Fresenius Kabi Awarded Breakthrough Technology Agreement With Premier, Inc. For the Ivenix Infusion System
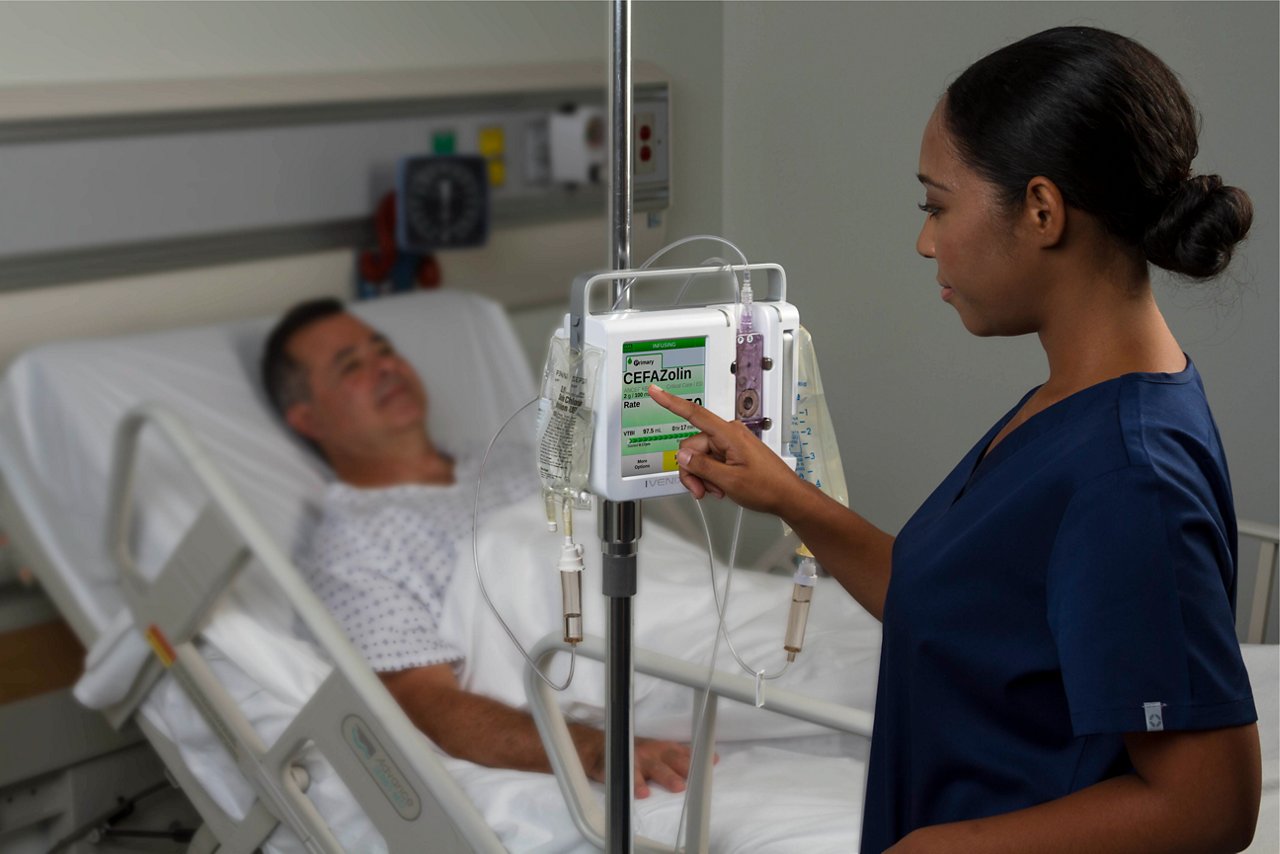
The Ivenix Infusion System is designed to overcome deficiencies in legacy pumps. The Ivenix patented pumping technology delivers critical medications with flow that is measured and controlled to help optimize safety. The pump operates unaffected by external conditions such as bag height, back pressure, and fluid viscosity to deliver accurate medication delivery, and it protects patients from uncontrolled flow.
The Ivenix Large-Volume Pump includes a large, smartphone-like touchscreen that allows clinicians to auto-program, review, and accept medication orders on the pump. The built-in integration engine within the Ivenix Infusion System supports secure interoperability with leading electronic medical records (EMR) systems. The Ivenix system from Fresenius Kabi also offers valuable tools to automate testing, reducing the implementation timeline and the resources needed to synchronize the drug library with the EMR formulary.
The Ivenix platform also facilitates enterprise-wide, centralized management of the entire pump fleet. This device command center allows for comprehensive oversight of the infusion pump fleet from device utilization to location awareness, proactive maintenance, user management, and remote software and security updates—without wasting valuable time hunting and gathering devices.
Premier, Inc. is a leading healthcare improvement company, uniting an alliance of more than 4,400 U.S. hospitals and health systems and approximately 250,000 other providers and organizations to transform healthcare. With integrated data and analytics, collaboratives, supply chain solutions, and consulting and other services, Premier enables better care and outcomes at a lower cost.
Fresenius Kabi is a global healthcare company that specializes in lifesaving medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used for the therapy and care of critically and chronically ill patients.
Its product portfolio comprises a range of highly complex biopharmaceuticals, clinical nutrition, medical technologies, and I.V. generic drugs. Within biopharmaceuticals, Fresenius Kabi offers, among others, biosimilar drugs with a focus on autoimmune diseases and oncology. The company’s clinical nutrition offering includes a wide selection of enteral and parenteral nutrition products. In the segment of medical technologies, its offering includes vital disposables, infusions pumps, apheresis machines, cell therapy devices, and more.
With its corporate mission of "caring for life", Fresenius Kabi puts essential medicines and technologies in the hands of people who help patients and finds the best answers to the challenges they face.
Following its “Vision 2026”, the company is furthermore committed to increase efficiencies in the therapy and care of patients and improve access to high-quality healthcare around the globe. Fresenius Kabi aspires to be leading globally in its product segments – all for the benefit of patients, its customers, and its stakeholders.
For more information, please visit www.fresenius-kabi.com.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g., changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius Kabi does not undertake any responsibility to update the forward-looking statements in this release.
Management Board: Pierluigi Antonelli (Chairman), Marc Crouton, John Ducker, Andreas Duenkel, Dr. Christian Hauer, Dr. Michael Schönhofen
Chairman of the Supervisory Board: Michael Sen
Registered Office: Bad Homburg, Germany
Commercial Register: Amtsgericht Bad Homburg - HRB 11654