Fresenius Kabi Introduces Leucovorin Calcium Injection, USP
Only liquid presentation available for Leucovorin Calcium Injection, USP
January 29, 2019
LAKE ZURICH, Ill., January 29, 2019 – Fresenius Kabi announced today the immediate availability in the United States of Leucovorin Calcium Injection, USP, a medication indicated to counteract the toxicity and diminish the effects of impaired methotrexate elimination.
Leucovorin Calcium is also used as a rescue drug after high-dose methotrexate therapy in osteosarcoma, in the treatment of megaloblastic anemia due to folic acid deficiency when oral therapy is not feasible, and in combination with 5-fluorouracil to prolong survival in the palliative treatment of patients with advanced colorectal cancer.
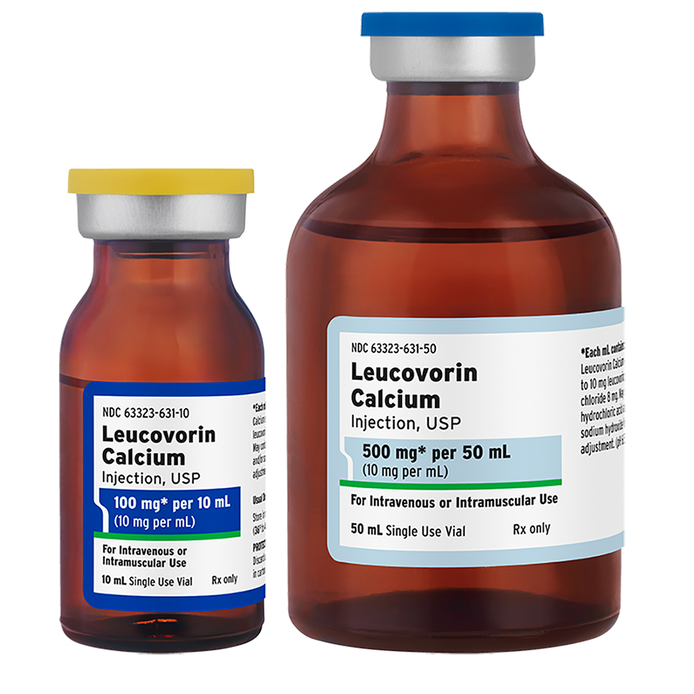
Fresenius Kabi Leucovorin Calcium Injection, USP is available in 10 mg per mL single-use vials, 100 mg per 10 mL and 500 mg per 50 mL strengths. This is the only liquid presentation of Leucovorin available in the United States.
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company is a leading developer, manufacturer and provider of injected and infused medicines in the United States with special expertise in producing high quality, affordable generic alternatives to more expensive brand-name drugs.
“At Fresenius Kabi we are dedicated to finding safe and affordable solutions for health care providers,” said John Ducker, president and CEO of Fresenius Kabi USA. “We are pleased to offer a version of Leucovorin Calcium Injection that eliminates the need for reconstitution, providing pharmacists an efficient way to prepare this medication.”
About Leucovorin Calcium Injection, USP
Leucovorin calcium rescue is indicated for intramuscular or intravenous administration after high dose methotrexate therapy in osteosarcoma. Leucovorin calcium is also indicated to diminish the toxicity and counteract the effects of impaired methotrexate elimination and of inadvertent over dosages of folic acid antagonists.
Leucovorin calcium is indicated in the treatment of megaloblastic anemias due to folic acid deficiency when oral therapy is not feasible.
Leucovorin is also indicated for use in combination with 5-fluorouracil to prolong survival in the palliative treatment of patients with advanced colorectal cancer. Leucovorin should not be mixed in the same infusion as 5-fluorouracil because a precipitate may form.
Important Safety Information
Leucovorin is improper therapy for pernicious anemia and other megaloblastic anemias secondary to the lack of vitamin B12. A hematologic remission may occur while neurologic manifestations continue to progress.
In the treatment of accidental over dosages of intrathecally administered folic acid antagonists, do not administer leucovorin intrathecally. LEUCOVORIN MAY BE HARMFUL OR FATAL IF GIVEN INTRATHECALLY.
Because of the calcium content of the leucovorin solution, no more than 160 mg of leucovorin should be injected intravenously per minute (16 mL of a 10 mg/mL, or 8 mL of a 20 mg/mL solution per minute).
Leucovorin enhances the toxicity of 5-fluorouracil. Although the toxicities observed in patients treated with the combination of leucovorin plus 5-fluorouracil are qualitatively similar to those observed in patients treated with 5-fluorouracil alone, gastrointestinal toxicities (particularly stomatitis and diarrhea) are observed more commonly and may be more severe and of prolonged duration in patients treated with the combination.
The concomitant use of leucovorin with trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection was associated with increased rates of treatment failure and morbidity in a placebo-controlled study.
Adverse reactions include: anaphylactoid reactions, urticaria, anaphylactic reactions (shock), leukopenia, thrombocytopenia, infection, nausea, vomiting, diarrhea, stomatitis, constipation, lethargy/malaise/fatigue, alopecia, dermatitis, and anorexia.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Excessive amounts of leucovorin may nullify the chemotherapeutic effect of folic acid antagonists.
Folic acid in large amounts may counteract the antiepileptic effect of phenobarbital, phenytoin and primidone, and increase the frequency of seizures in susceptible pediatric patients.
This Important Safety Information does not include all the information needed to use Leucovorin Calcium Injection, USP safely and effectively. Please see the full prescribing information for LEUCOVORIN CALCIUM INJECTION, USP. Full prescribing information is also available at https://tinyurl.com/y7g5qbt3.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany.
