Fresenius Kabi Introduces Palonosetron Hydrochloride Injection
Alternative to ALOXI® Now Available
April 11, 2018
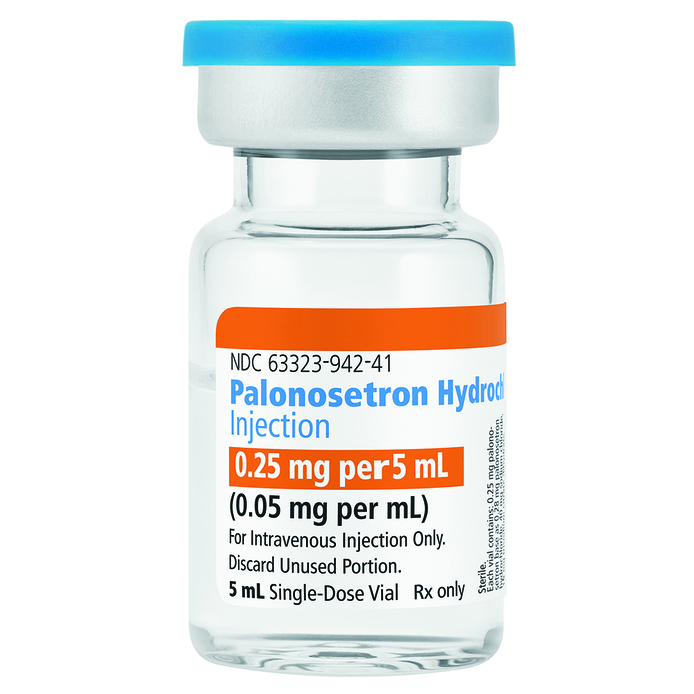
LAKE ZURICH, Ill., April 11, 2018 – Fresenius Kabi announced today the immediate availability in the United States of Palonosetron Hydrochloride Injection. Fresenius Kabi Palonosetron Hydrochloride Injection is available in a 0.25 mg per 5 mL single dose vial.
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition.
“The addition of Palonosetron to the injectable antiemetic medicines offered by Fresenius Kabi is the latest example of our commitment to offer a large product portfolio to meet the diverse needs of clinicians and patients,” said John Ducker, president and CEO of Fresenius Kabi USA.
About Palonosetron Hydrochloride Injection
Palonosetron Hydrochloride (HCl) Injection is a 5-HT3 serotonin receptor indicated in adults for moderately emetogenic cancer chemotherapy – prevention of acute and delayed nausea and vomiting associated with initial and repeat courses; highly emetogenic cancer chemotherapy – prevention of acute nausea and vomiting associated with initial and repeat courses; and prevention of postoperative nausea and vomiting (PONV) for up to 24 hours following surgery. Efficacy beyond 24 hours has not been demonstrated.
Important Safety Information
Palonosetron HCl Injection is contraindicated in patients with hypersensitivity to palonosetron or any of its components (e.g., anaphylaxis).
Serotonin Syndrome: Development of serotonin syndrome has been reported with 5-HT3 receptor antagonists alone but particularly with concomitant use of serotonergic drugs. If symptoms of serotonin syndrome occur, discontinue Palonosetron HCl Injection.
The most common adverse reactions in chemotherapy-induced nausea and vomiting (≥5%) are headache and constipation.
The most common adverse reactions in postoperative nausea and vomiting (≥2%) are QT prolongation, bradycardia, headache, and constipation
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use Palonosetron Hydrochloride Injection safely and effectively. Please see full prescribing information for Palonosetron Hydrochloride Injection at https://tinyurl.com/y9hnc2bp or www.fresenius-kabi.com/us.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany.
