Temsirolimus Injection Now Available from Fresenius Kabi
Newest addition to the most comprehensive injectable oncology portfolio in America
May 21, 2021
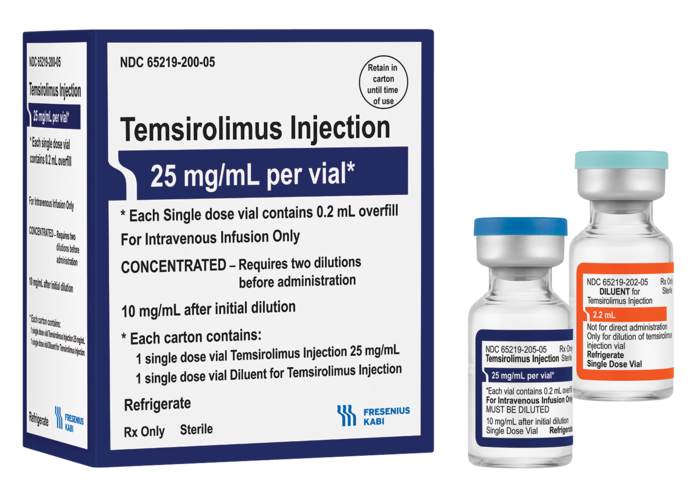
LAKE ZURICH, Ill., May 21, 2021 – Fresenius Kabi announced today the immediate availability of Temsirolimus Injection in the United States. Fresenius Kabi’s Temsirolimus Injection is supplied as a kit including one vial of 25 mg/mL Temsirolimus solution and one vial of diluent.
Temsirolimus Injection, a generic version of the brand Torisel®, is used to treat advanced renal cell carcinoma. It is the newest addition to the most comprehensive injectable oncology portfolio in America.
“Fresenius Kabi is committed to expanding affordable treatment options for patients,” said John Ducker, president and CEO of Fresenius Kabi USA. “The addition of Temsirolimus is the most recent example of our development program and deep expertise in oncology medicines. Fresenius Kabi is pleased to continue to develop high-quality therapies that clinicians can deliver knowing they are safe, efficacious and accessible for their patients.”
Fresenius Kabi is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company offers a broad portfolio of generic injectable oncology medicines and has a pipeline that includes biosimilars and complex generics for oncology patients.
To learn more about Fresenius Kabi, including its expanding U.S. centers for pharmaceutical research, manufacturing and distribution, please visit www.fresenius-kabi.com/us.
About Temsirolimus Injection
Temsirolimus Injection is indicated for the treatment of advanced renal cell carcinoma.
Important Safety Information
Temsirolimus Injection is contraindicated in patients with bilirubin > 1.5×ULN.
Hypersensitivity/Infusion Reactions (including some life-threatening and rare fatal reactions) can occur early in the first infusion of Temsirolimus Injection. Patients should be monitored throughout the infusion.
To treat hypersensitivity reactions, stop Temsirolimus Injection and treat with an antihistamine. Temsirolimus Injection may be restarted at physician discretion at a slower rate.
Hepatic Impairment: Use caution when treating patients with mild hepatic impairment and reduce dose.
Hyperglycemia and hyperlipidemia are likely and may require treatment. Monitor glucose and lipid profiles.
Infections may result from immunosuppression.
Monitor for symptoms or radiographic changes of interstitial lung disease (ILD). If ILD is suspected, discontinue Temsirolimus Injection, and consider use of corticosteroids and/or antibiotics.
Bowel perforation may occur. Evaluate fever, abdominal pain, bloody stools, and/or acute abdomen promptly.
Renal failure, sometimes fatal, has occurred. Monitor renal function at baseline and while on Temsirolimus Injection.
Due to abnormal wound healing, use Temsirolimus Injection with caution in the perioperative period.
Proteinuria and nephrotic syndrome may occur. Monitor urine protein prior to the start of Temsirolimus Injection therapy and periodically thereafter. Discontinue Temsirolimus Injection in patients with who develop nephrotic syndrome.
Live vaccinations and close contact with those who received live vaccines should be avoided.
Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of the potential hazard to the fetus and to use effective contraception.
Elderly patients may be more likely to experience certain adverse reactions, including diarrhea, edema and pneumonia.
The most common adverse reactions (incidence ≥ 30%) are rash, asthenia, mucositis, nausea, edema, and anorexia. The most common laboratory abnormalities (incidence ≥30%) are anemia, hyperglycemia, hyperlipidemia, hypertriglyceridemia, elevated alkaline phosphatase, elevated serum creatinine, lymphopenia, hypophosphatemia, thrombocytopenia, elevated AST, and leukopenia.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1‐800‐FDA‐1088 or www.fda.gov/medwatch.
Strong inducers of CYP3A4/5 and inhibitors of CYP3A4 may affect concentrations of the primary metabolite of Temsirolimus Injection. If alternatives cannot be used, dose modifications of Temsirolimus Injection are recommended.
Lactation: Do not breastfeed.
This Important Safety Information does not include all the information needed to use Temsirolimus Injection safely and effectively. Full prescribing information is also available at www.fresenius-kabi.com/us.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. To learn about U.S. career opportunities at Fresenius Kabi, visit us at https://www.fresenius-kabi.com/us/join-us.
###
Torisel® is a registered trademark of Pfizer Inc.
