Anesthesia and Analgesia

Fresenius Kabi offers one of the largest injectable anesthesia portfolios in the United States, including general and local anesthetics, neuromuscular blocking and reversal agents, NSAIDs, and anxiolytics; ensuring providers have continuous access to the medications they need to keep patient care running smoothly.
Featured Products
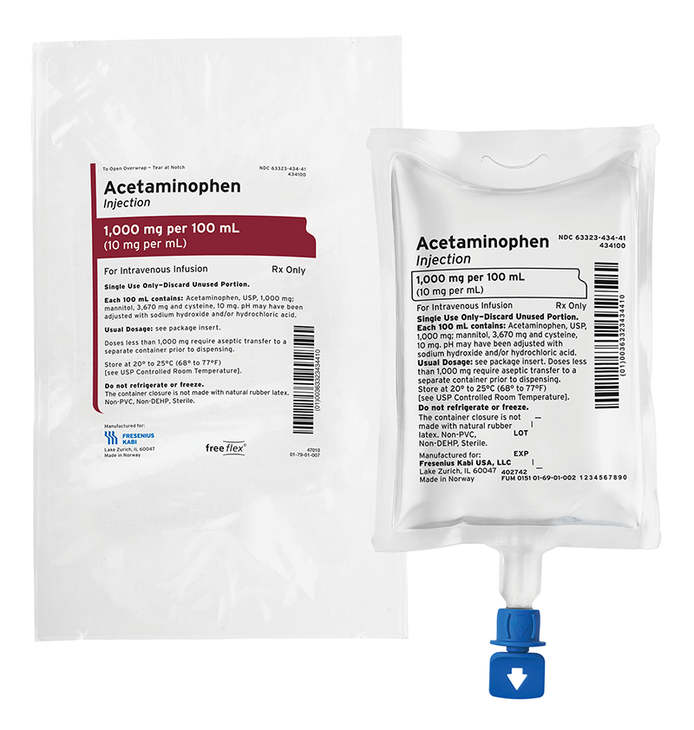
Acetaminophen Injection
Fresenius Kabi now provides Acetaminophen Injection for intravenous use in Ready-to-Use freeflex® bags. As a leader in infusion and nutrition therapy, Fresenius Kabi is one of the largest manufacturers of IV bags in the world. The unique port design promotes safety and convenience.
Acetaminophen is an alternative option for multimodal pain management in the perioperative period1.
To place an order, contact your Sales Representative or call Customer Service at 1.888.386.1300 | www.fresenius-kabi.com/us
1Multimodal Analgesia and Alternatives to Opioids for Postoperative Analgesia. Veena Graff, MD; Taras Grosh, MD. October 2018

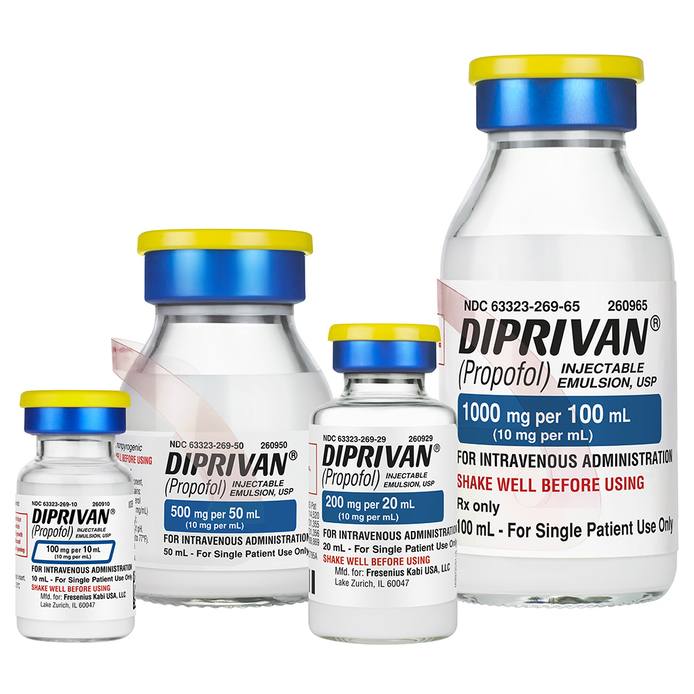
Diprivan®
As the manufacturer of Diprivan, the #1 prescribed propofol in the U.S. — and the only FDA approved propofol containing the antimicrobial retardant EDTA — Fresenius Kabi is committed to both helping meet demand when the market’s supply is challenged and innovating to address unmet clinical needs. With multiple manufacturing sites to guard against supply interruption and product introductions such as the market-exclusive 10 mL Diprivan presentation that helps improve workflow efficiency and reduces waste in low-dose procedures, Diprivan continues to be there when patients need it.
For more information visit the Diprivan website
Indications
DIPRIVAN (Propofol) Injectable Emulsion is an intravenous sedative-hypnotic agent for use in the induction and maintenance of anesthesia or sedation.
Safety Information
DIPRIVAN Injectable Emulsion is contraindicated in patients with a known hypersensitivity to DIPRIVAN Injectable Emulsion or any of its components, and also in patients with allergies to eggs, egg products, soybeans or soy products. Use of DIPRIVAN Injectable Emulsion has been associated with both fatal and life-threatening anaphylactic and anaphylactoid reactions, as well as Propofol Infusion Syndrome. For general anesthesia or MAC sedation, DIPRIVAN Injectable Emulsion should be administered only by persons trained in the administration of general anesthesia and not involved in the conduct of the surgical/diagnostic procedure.
For sedation of intubated, mechanically ventilated patients in the ICU, DIPRIVAN Injectable Emulsion should be administered only by persons skilled in the management of critically ill patients and trained in cardiovascular resuscitation and airway management. Sedated patients should be continuously monitored. Strict aseptic technique must always be maintained during handling.
DIPRIVAN Injectable Emulsion is a single access parenteral product. Failure to use aseptic technique has been associated with microbial contamination of the product, including fever, infection/sepsis, other life-threatening illness and/or death. There have been reports, in the literature and other public sources, of the transmission of bloodborne pathogens (such as Hepatitis B, Hepatitis C, and HIV) from unsafe injection practices, and use of Propofol vials intended for single use on multiple persons. DIPRIVAN Injectable Emulsion vials are never to be accessed more than once or used on more than one person.
Please see Full Prescribing Information
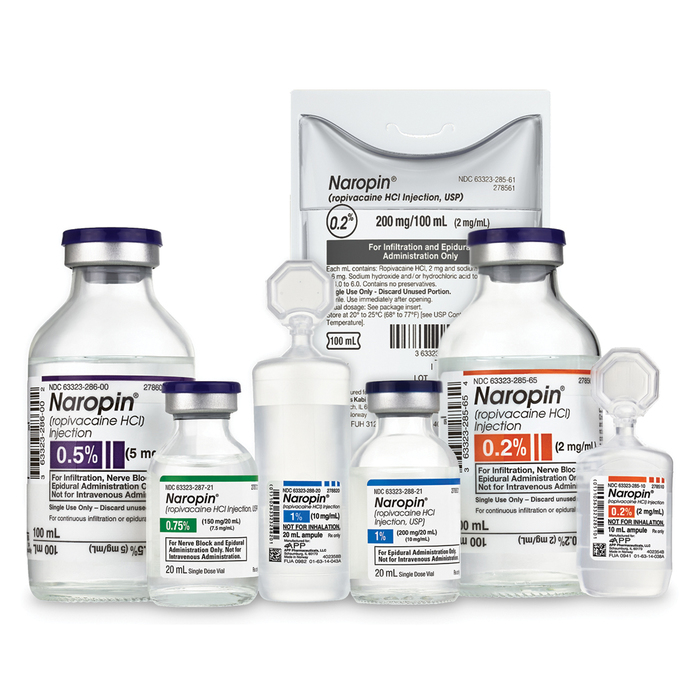
Naropin
Naropin offers an extensive portfolio of concentrations and delivery systems including freeflex bags, single dose infusion bottles, plastic ampule steri-paks, and single dose vials. Why Compromise? Choose Naropin for Surgical Anesthesia, Acute Pain Management and Labor & Delivery.
For more information visit the Naropin website
INDICATIONS AND USAGE
Naropin is indicated for the production of local or regional anesthesia for surgery and for acute pain management. Surgical Anesthesia: epidural block for surgery including cesarean section; major nerve block; local infiltration. Acute Pain Management: epidural continuous infusion or intermittent bolus, eg, postoperative or labor; local infiltration.
Contraindications
Naropin is contraindicated in patients with a known hypersensitivity to ropivacaine or to any local anesthetic agent of the amide type.
Important Safety Information
Using NAROPIN beyond recommended doses to increase motor block or duration of sensory block may negate its favorable cardiovascular advantages, in the event that an inadvertent intravascular injection occurs.
NAROPIN may be associated with adverse reactions which are characteristic of those associated with other amide-type local anesthetics. In clinical trials, side effects were mild and transient and may reflect the procedures, patient health status, and/or other medications used.
Adverse events reported at a rate of ≥5%: hypotension, nausea, vomiting, bradycardia, fever, pain, postoperative complications, anemia, paresthesia, headache, pruritus, and back pain.
There have been adverse event reports of chondrolysis in patients receiving intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures. NAROPIN is not approved for this use.
For full prescribing information, please see package insert.
